


Revolutionized is reader-supported. When you buy through links on our site, we may earn an affiliate commision. Learn more here.
The periodic table of elements contains the building blocks that make up all the objects in the world. For example, water is a combination of hydrogen and oxygen atoms. All living things contain carbon atoms. As of 2023, the periodic table contains 118 unique elements. Scientists are trying to create new elements by using particle accelerometers, but this can be a tricky process. Once you’re familiar with the element key, layout and structure, it’s easy to read the periodic table.
Here is a brief overview of what you need to get started. These tidbits will lay the foundation for exploring the periodic table’s finer details.
The periodic table of elements is a chart that organizes all known natural and human-made elements into clusters. These are rows and columns, arranged in ascending order by atomic number, which represents the number of protons. Each period, or row, contains elements with the same number of electrons. The position of each element on the table can tell you its properties, such as orbital arrangement or chemical composition.
The periodic table is organized with names and symbols. For a more in-depth explanation, please see below. In short, the rows are referred to as periods, and the columns are called groups.
The chart contains two parts. The bulk of the table contains the known naturally occurring elements, while a section of two rows below represents a few things. The top row is the lanthanoids and the bottom is the actinoids. The lanthanoids are rare-earth elements, and the actinides are radioactive.
Additionally, elements 95-118 are ones that people have created, mostly in laboratory settings. These are known as synthetic elements. Theoretically, this means the table could change over time as chemists and other innovators discover new elements. However, many of these that have been discovered do not have any practical applications. This applies to elements 99 and higher.
These are separated to keep the table manageable in size. Adding these elements to the base structure would make it too wide or unruly.
The originator of the modern periodic table was Dmitri Mendeleev, as he was the first person to organize elements by a specific characteristic — atomic weight. This is the format we are used to seeing today, though Alexandre-Emile Béguyer de Chancourtois in 1862 wrote a paper about similar topics. Because he was a geologist, people initially dismissed the legitimacy of this research until Mendeleev later affirmed it. The table was created, even though humans didn’t know the full story about an atom’s structure yet!
The table is called periodic for more reasons than the fact that it includes rows called periods. The rows represent repeated, or periodic, traits within elements. Periodic is used to describe the table here in the same way one would describe sunsets — they are cyclical and repeatable, therefore, periodic.
You know by looking at the table that elements near each other are likely to have the same hardness or reactivity. The name implies there is consistency and dependability in the elements’ attributes.
Additionally, this also informed the Periodic Law, which describes the phenomenon of arranging elements. The law states that when elements are arranged by their atomic number, they demonstrate periodic tendencies, or repeated behaviors of their physical and chemical properties.
The box containing each element’s information is known as the element key. Each key contains an element’s name, unique symbol, atomic weight and atomic number. Oxygen, for example, has an atomic number of 8, an atomic weight of 15.996 and a unique symbol, O. What do each of these sections on the element key tell us?
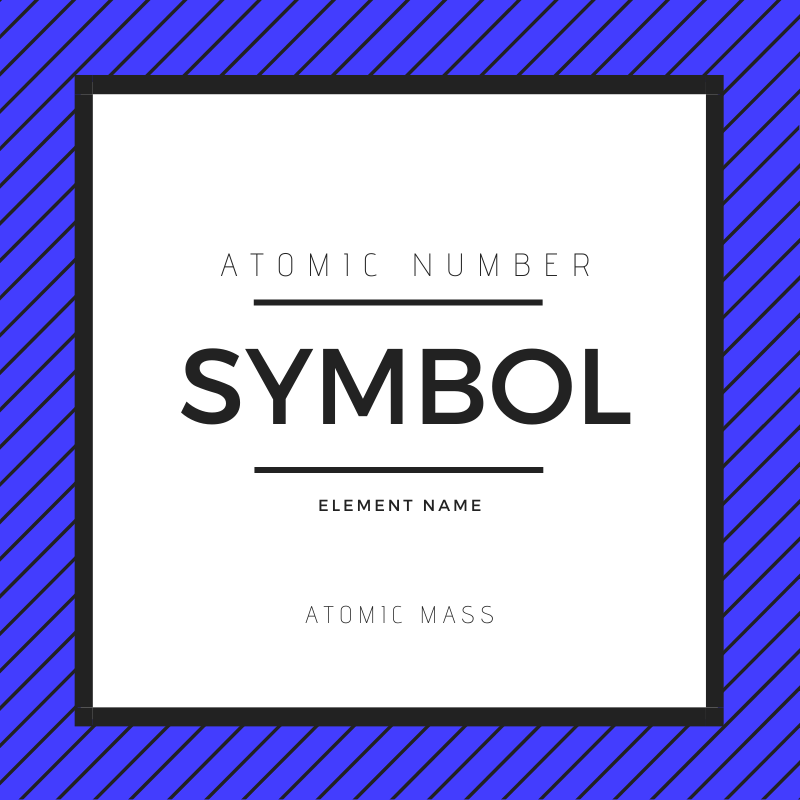
An element’s atomic number lets you know how many protons are in the nucleus. Since oxygen is number 8 on the periodic table, that tells you there are eight positive protons in the center of each atom. The number of protons in an element cannot change or else it would become a different element entirely!
Every element on the periodic table has an atomic number assigned to it. Not only does it let you count the protons, but it also determines the element’s place on the table. Each element has one more proton than the one before it.
It’s worth noting that most atoms have an equal number of protons and electrons, so the atomic number gives you a hint as to how many electrons an atom contains. However, this is only sometimes the case. Some atoms have more electrons than protons, giving them a negative charge. Others have fewer electrons than protons, giving them a positive charge.
These positively and negatively charged atoms are called ions. Cations are positively charged, while anions have a negative charge. Therefore, don’t assume how many electrons an element has based on its atomic number on the periodic table. That just tells you the number of protons.
The atomic mass is the number usually — but not always — located at the bottom of an element key. This number tells you how much a single atom of that element weighs in atomic mass units. If you read the periodic table, you’ll see that oxygen weighs 15.999 u.
However, even two atoms of the same element might have different weights. That’s because they sometimes contain different numbers of neutrons. Just as an ion is an element with more or fewer electrons than usual, an isotope is a version of an element that contains a different number of neutrons.
Neutrons have mass. Therefore, different isotopes will have different amounts of mass. The atomic mass number on the modern periodic table is the weighted average of the atomic masses of that element’s naturally occurring isotopes. In other words, the number you’re seeing is the element’s average mass, but you might find it at a different mass depending on the number of neutrons it contains. That’s why many atomic mass numbers have a decimal.
If you simply want to know an element’s mass number — which doesn’t have a decimal, because it’s the actual number of protons and neutrons in an atom — round the atomic mass to the nearest whole number. For example, beryllium’s atomic mass is 9.0112, so you can round it to nine. That’s the total number of neutrons and protons combined in the nucleus of a beryllium atom.
From there, you can find the number of neutrons in an atom by subtracting the total number of protons from the mass number. Remember that the atomic number tells you how many protons there are. Beryllium is number four on the periodic table, so it has four protons. Nine total protons and neutrons minus four protons leaves you with five neutrons. So, beryllium has four protons and five neutrons. That’s why its mass number is nine.
Scientists created elements 93 through 118 in a laboratory. These artificial elements don’t exist naturally and haven’t survived longer than several milliseconds at a time. The atomic weight of these elements is based on the element that survived longest when it was created. Future research can change the listed atomic weight of these elements.
As you read the periodic table from left to right, atomic mass tends to increase.
Finally, an element’s name determines the atom’s symbol. In most cases — like oxygen or carbon — it’s simply the first letter of the element’s name. In others — like potassium or mercury — scientists have to look back into history.
Potassium has the designation K because of its Neo-Latin name, kalium. Mercury has the designation Hg because of its Greek name, hydrargyrum, which means “liquid silver.” Silver, meanwhile, has the designation Ag for its Latin name, argentum. “Argentum” later became the French word for the same color — argent. How many elements can you guess just by looking at their atomic symbol?
The structure of the modern periodic table might seem strange to the casual observer, but its design serves a purpose. Each element is placed according to its atomic weight and number. The vertical columns are groups, and the horizontal rows are periods.
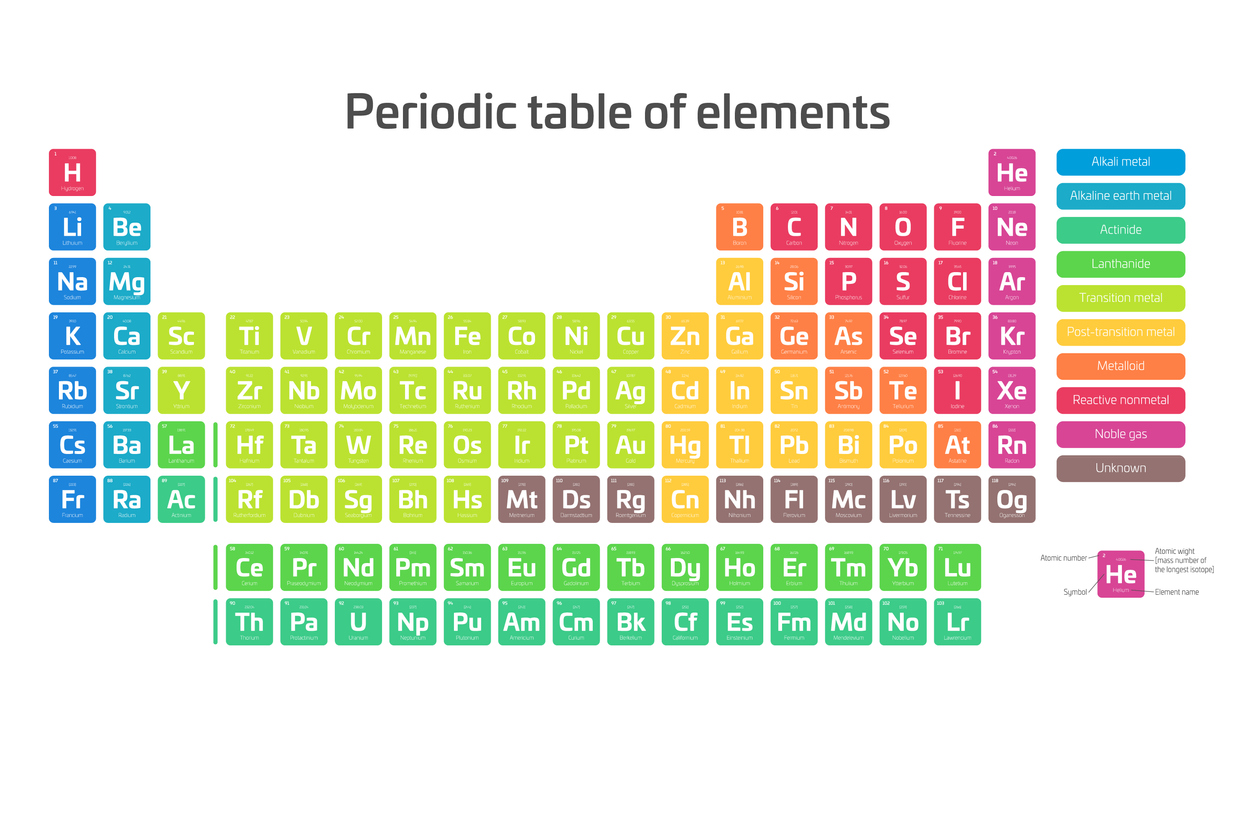
Elements are grouped into columns based on their chemical properties. A Roman numeral — or, in the modern periodic table, an Arabic numeral — designates each column. This is the number of electrons an element has in its outer shell, which are known as valence electrons.
Valence electrons are the number of electrons an element has available to bond with other elements to make molecules.
Group 1 consists of Alkali Metals — things like sodium, potassium,and cesium, which are not as dense as other metals and are highly reactive. Some of them even explode when they come into contact with water or air.
Group 2 includes the Alkaline Earth Metals like calcium and magnesium, which bond easily. Their atoms are smaller than those in Group 1.
Groups 3 through 12 are the Transition Metals. This group makes up most of the periodic table. On the far right, you’ll find some metals as well as metalloids and nonmetals. The most common nonmetals are Group 17, the Halogens, and Group 18, the Noble Gases, which don’t bond with anything.
When you read a group from top to bottom, it’s known as “reading down a group.”
Alkali Metals | Alkaline Earth Metals | Transition Metals | Actinides | Lanthanides | Basic Metals | Metalloids | Nonmetals | Halogens | Noble Gases
Surrounding each element’s nucleus is a shell, also called an orbital, that can only hold a certain number of electrons. Elements in the periodic table are broken down into rows based on the number of shells they have. In other words, all the elements in a period have the same number of atomic orbitals.
Neon is in the second row, or period, which means it has two shells. The first shell can only hold two electrons, while the second shell can hold up to eight electrons, for a total of 10.
On the bottom of the periodic table, you’ve got the lanthanides and actinides. Lanthanides all have an atomic number larger than that of lanthanum and are almost all soft, silvery metals. Actinides are in the very bottom row of the periodic table, and they all have atomic numbers higher than that of actinium. Every one of these elements is highly radioactive. Scientists created most of the actinides in a lab.
To make it easier to read the periodic table, modern versions include color-coding. Each color breaks elements into their different groups. Elements 57 through 71 and 89 through 103 are in separate rows to improve readability as well.
Once you learn how to read the periodic table, it becomes easier to see the trends that are presented there. For example, if you follow the table from left to right, you’ll see that electronegativity increases. Electronegativity occurs when the valence shell carrying the electrons is less than half full.
Moving from the top to the bottom shows a decrease in electronegativity. This doesn’t apply to noble gasses — which always have a full valence shell — or the lanthanides and actinides at the bottom of the table.
Going from right to left shows an increase in atomic radius, or the distance between the element’s nucleus and its valence shells. The group one elements have the largest radius, while the noble gasses have the smallest.
This radius also increases as you move from the top of the table to the bottom. Moving in this direction shows increasing metallic characteristics — the further left you get on the chart, the more metallic the elements become. Noble gasses — which are distinctly non-metallic — are on the far right and alkaline metals are on the far left.
The periodic table reveals even more aspects of the element to viewers. In addition to electronegativity, you can discover first ionization energy, electron configuration and oxidation states. What do these mean and how do they give you more insight into the element?
First ionization energy tells scientists how much energy is required to eliminate the highest-energy electron from neutral atoms. This only applies to gases. The goal of first ionization energy is to make moles, or kilojoules, of gaseous ions with increasing charges. How can you tell this quality by looking at the periodic table?
The first ionization energy typically increases going from the bottom of a group to the top and while going from the left of a period to the right. Elements at the top and right of the table require more energy to separate its larger parts.
Electron configuration describes the structure of the atomic orbitals present in the element. Each block has a scaling number of electrons. The periodic table has four orbital blocks, including:
This matters because its energy determines the element’s properties. Each element in the block increases in energy and configuration complexity as you progress through the groups.
How can you tell an element’s electron configuration by looking at the table? The first and second group comprise the s-block, with hydrogen and helium labeled as 1s1 and 1s2, respectively. The first number denotes energy level, the “s” explains the orbital type, and the exponent value indicates electron count. The element’s placement in the period determines its energy level in ascending order, with lithium’s configuration being 1s22s1, including the lower orbital first. To read the rest of the table, note:
This determines how many electrons an atom can lose or gain before it becomes unstable. Otherwise, it stays in a sturdy, ionic state. Its state increases when it loses and decreases when it gains. Every element has an oxidation number that informs you the electrical charge required to render it ionic. The numbers range from -2 to +2. Can you see this on the periodic table?
Not all tables feature them in plain text, but there are easy-to-follow rules to know where to find elements with specific oxidation numbers:
The Periodic Table song lyrics clearly spell out every element of the table, making it an engaging and effective tool for memorization. Singing along can help you recall each component in order, enhancing your understanding of their placement and solidifying their names and symbols in your memory.
The song gained popularity through various adaptations, with one of the most famous versions composed by Tom Lehrer in 1959. The American mathematician and satirist created a lively melody that lists the elements in order of their atomic number. Since then, many versions have emerged, each with its own unique style and flair.
Check out the recent version of the Periodic Table song, which now includes the four new elements added in the last decade. It also serves as a mnemonic device and can add fun to learning about chemistry. Feel free to make up your own variations of the lyrics or create new verses that incorporate fun facts about specific elements.
You can easily download a printable periodic table if you prefer a physical reference. This handy resource is perfect for you—whether you’re a student, teacher, or chemistry enthusiast—who wants quick access to essential information. Print it out and hang it in your classroom, study area or wherever you find it most useful.
Learning how to read the periodic table key and its components is not exclusive to scientists and chemists. Knowing how much information is packed in these colorful tiles reveals countless insights into the world around us. The profound organizational methods of the inventors has allowed the best minds in science to make new discoveries, invent technologies and make the world a better place to live. By using the guide above, you’ll be able to read the periodic table with ease in no time!
Revolutionized is reader-supported. When you buy through links on our site, we may earn an affiliate commision. Learn more here.


This site uses Akismet to reduce spam. Learn how your comment data is processed.