The Wonderful Reason Why Fireworks Get Their Color
June 28, 2019 - Emily Newton
Revolutionized is reader-supported. When you buy through links on our site, we may earn an affiliate commision. Learn more here.
Fireworks stands have started popping up on every corner, and you know what that means – it’s nearly the 4th of July! Fireworks have come a long way from the rockets’ red glare that John Stafford Smith wrote about in the Star-Spangled Banner. Now you can find fireworks in nearly every color of the rainbow, plus a few that might not even have names yet. Let’s take a look at the science of fireworks and how they get those amazing colors out of a simple explosion.
The History of Fireworks
Fireworks aren’t a new invention – it’s estimated that they’ve been around since the 2nd century BC when individuals in China discovered that bamboo, when thrown in the fire, explodes with a loud bang due to the air pockets within the wood. At the time, people thought these explosions would chase away any evil spirits that were lingering nearby.
It wasn’t until sometime between 600 and 900 AD that people added chemicals to the mix. They poured early gunpowder, a carefully crafted mixture of charcoal, sulfur and potassium nitrate into bamboo sticks or paper tubes to create the first fireworks. We’ve come so far since then… well, at least we don’t use bamboo sticks anymore.
The Science of Fireworks
We all know how explosions work – take fuel, add fire… boom! But how do fireworks work? To figure that out, we need to start with the chemicals used in fireworks?
The colorful celebratory explosions that we all know and love require four primary ingredients – an oxygen producer, a fuel for ignition, a binder and a chemical that produces the color. Since the fuel is pretty much the same in fireworks of every color, we’re going to go ahead and leave that out.
At the core of this formula is gunpowder or black powder, which hasn’t changed much from the original recipe used by the Chinese all those centuries ago. However, it might be combined a little better today.
The fuse that lights the propellant also continues to burn as the firework heads skyward. Once this fuse reaches the interior shell of the firework, containing the four ingredients mentioned above, it ignites. The result is a colorful explosion.
This isn’t the only answer to the question of what chemicals are used in fireworks. Now we get down to the really fun part – the colors!
Explosive Colors
A firework wouldn’t be a firework without an explosive burst of color at the end. To create these colorful displays, chemists need to know one important thing – what metals burn what colors.
The exact chemicals for each color of firework might vary, depending on manufacturing location and which ingredients are readily available. Typically, though, these displays depend on metal salts.
What are metal salts? Metal salts form when a metal replaces one hydrogen atom in an acid. People make them by mixing metal, acid and potash to create a dry, easily transportable substance that will burn the color of the metal included in the mixture. Some common colors and their associated metals are:
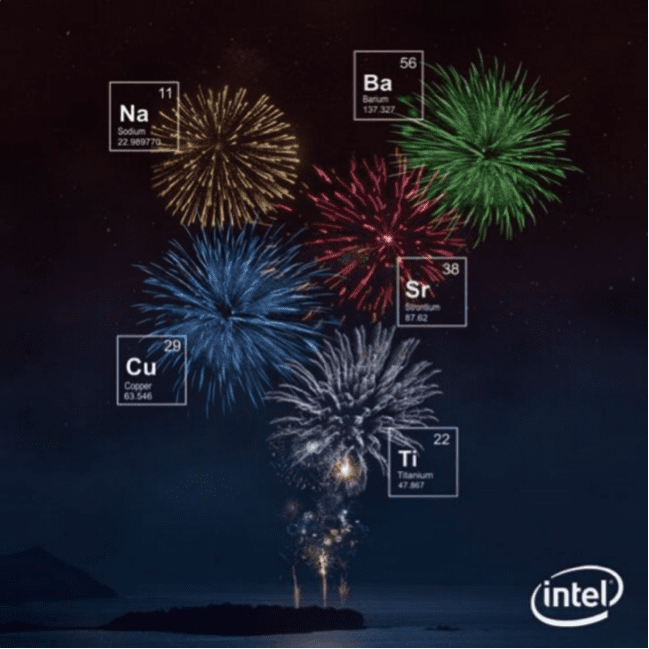
- Strontium carbonate, which burns red
- Calcium chloride, which burns orange
- Sodium nitrate, which burns yellow
- Barium chloride, which burns green
- Copper chloride, which burns blue
Chemists make other colors, like purple, with a mixture of copper and strontium, because – as you may remember your kindergarten art class – red and blue makes purple. Bright white fireworks contain metals such as magnesium. Burning magnesium, titanium or aluminum can also create silver fireworks, while iron or charcoal burn gold.
We wouldn’t suggest trying to make your own fireworks at home. It might sound easy, but the science of fireworks is a lot more complicated than it seems. Getting one part of the ratio wrong can cause the firework to fizzle out before it can launch or explode dangerously on the ground.
When you go out to enjoy a local pyrotechnics display, keep this in mind – we wouldn’t have these amazing and beautiful displays without some ancient Chinese chemists who accidentally discovered gunpowder and the magic of metal salts. If someone asks you what metal salts are, you have the answer. The science behind fireworks is a great thing to talk about while you’re watching those rockets’ strontium glare!
Revolutionized is reader-supported. When you buy through links on our site, we may earn an affiliate commision. Learn more here.
Author
Emily Newton
Emily Newton is a technology and industrial journalist and the Editor in Chief of Revolutionized. She manages the sites publishing schedule, SEO optimization and content strategy. Emily enjoys writing and researching articles about how technology is changing every industry. When she isn't working, Emily enjoys playing video games or curling up with a good book.